Our Stories
Our Stories
So, what are biologics and how are they different from traditional chemical medicines?
Traditionally, development of medicine centered around the synthesis of small molecules in a laboratory. These are relatively simple (think of aspirin), consist of very few atoms and are usually taken orally. They can easily be produced in substantial quantities.
Biologics are produced in living systems3 and due to this there is some inherent variability4,5 in the characteristics of the final drug produced. Variability has been observed in different batches of the same biologic medicine because of the size of the molecule, and the method of manufacture. Because of this, the manufacturing process is more complicated than it would be for a small molecule drug. 6
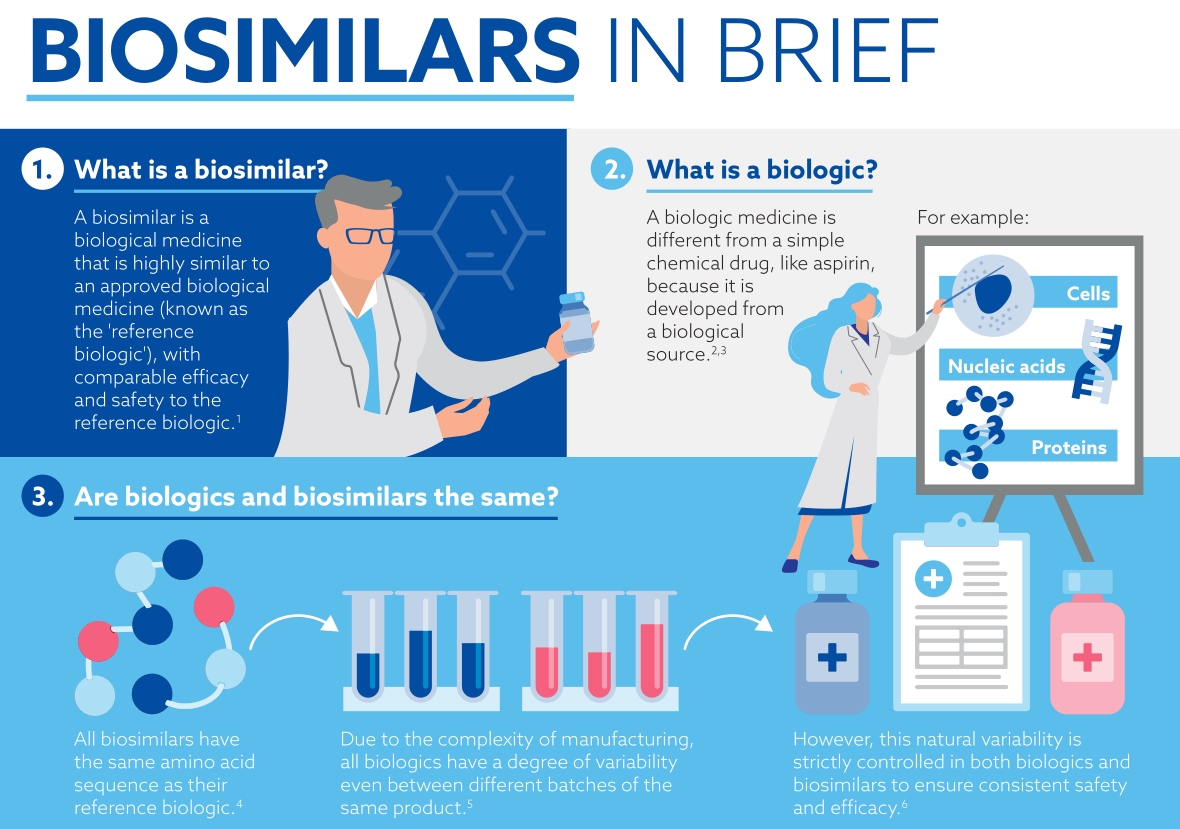

Then what are biosimilars and how are they tested?
Biosimilars are biological medicines that are highly similar to an approved biological medicine (known as the 'reference biologic'), with comparable efficacy and safety to the reference biologic.7 Biosimilars are different to generics, which are exact copies of original simple chemical drugs.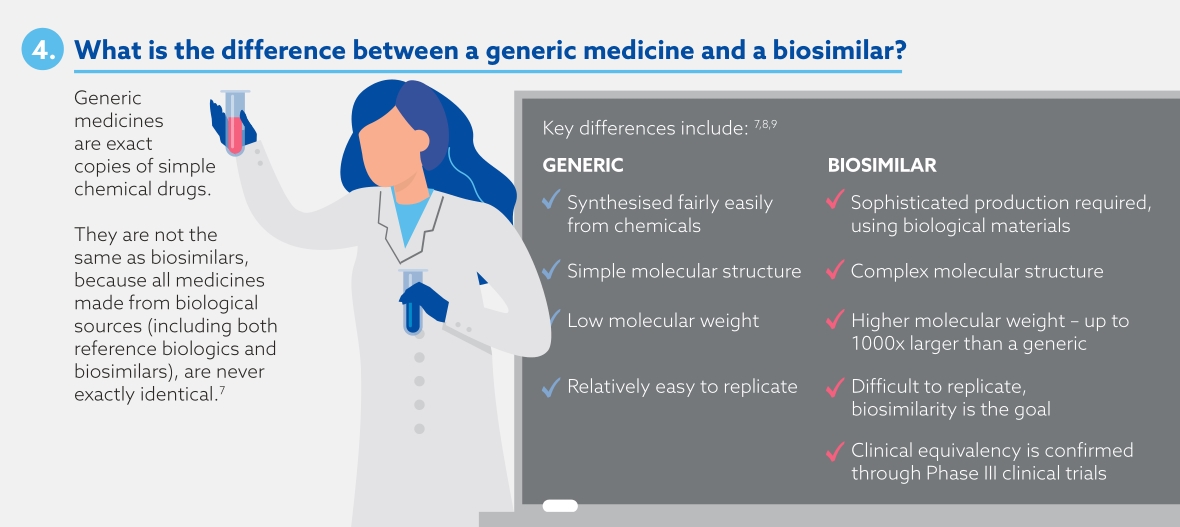

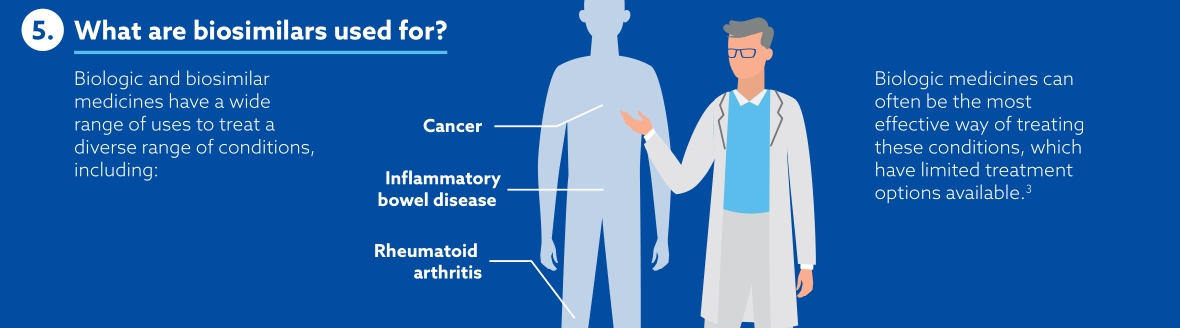

What does biosimilars mean to Samsung Bioepis?
Samsung Bioepis view biosimilars as key players in the world of medicines. By keeping costs low, making medicines available for more patients and offering more options for clinicians, payers and other healthcare professionals, biosimilars are shifting the paradigm of biologics. The number of biosimilars in development and in production are ever-increasing, maintaining healthy competition in the medicine market and offering high-quality alternatives.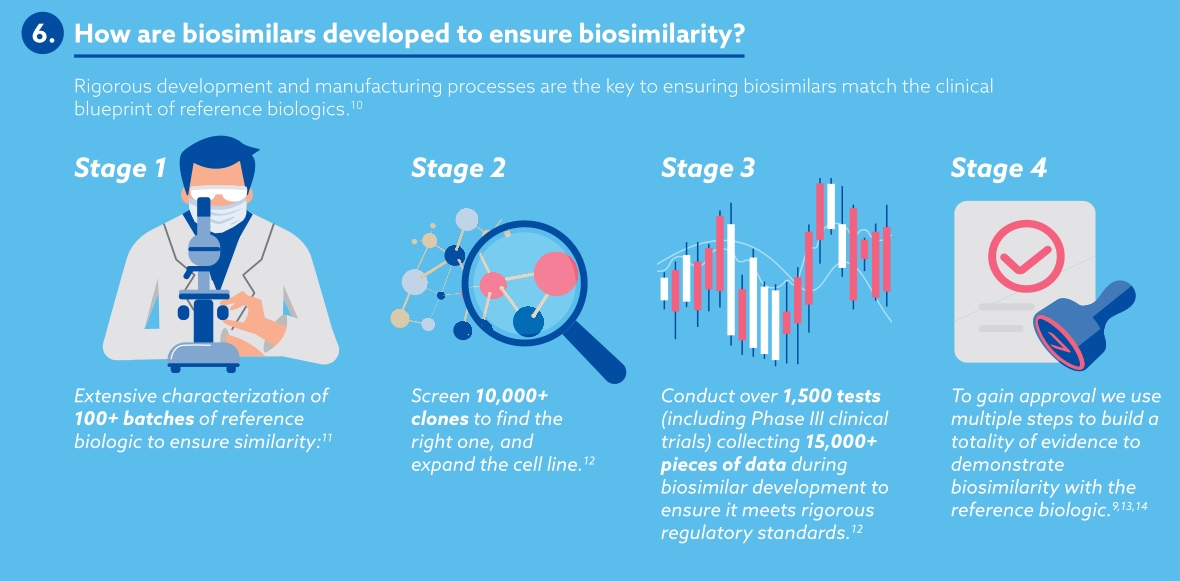

References
- 1. Tinsley SM, et al. Potential of biosimilars to increase access to biologics: considerations for advanced practice providers in oncology. Journal of the advanced practitioner in oncology. 2018 Nov;9(7):699.
- 2. IQVIA. 2018. The Impact of Biosimilar Competition in Europe.
- 3. Introduction to Biologics. International Alliance of Patients’ Organizations. 2018. Available from: https://www.iapo.org.uk/sites/default/files/files/factsheet1.pdf [Accessed April 2020]
- 4. Biological medicines: what you need to know about manufacturing? ABPI. British Biosimilars Association. Available from: https://www.abpi.org.uk/media/4570/biological-medicines-manufacturing-factsheet.pdf [Accessed April 2020]
- 5. Leung LK et al. What do oncologists need to know about biosimilar products? Chin J Cancer. 2016;35(1):91
- 6. Jurczak W, et al. Rituximab biosimilars for lymphoma in Europe. Expert opinion on biological therapy. 2019 Oct 3;19(10):1045-56.
- 7. European Medical Agency. Biosimilar medicines: Overview. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview [Accessed April 2020]
- 8. Christl L. FDA’s overview of the regulatory guidance for the development and approval of biosimilar Products in the US. Available from: https://www.fda.gov/files/drugs/published/FDA%E2%80%99s-Overview-of-the-Regulatory-Guidance-for-the-Development-and-Approval-of-Biosimilar-Products-in-the-US.pdf [Accessed April 2020]
- 9. Biogen’s Q4 & Full Year 2019 Financial Results and Business Update. Available at: https://investors.biogen.com/static-files/ce31eed8-8862-4bec-a63f-77c0fd6e15a1 +
References in IMG
- 1. European Medical Agency. Biosimilar medicines: Overview. Available from: https://www.ema.europa.eu/en/human-regulatory/overview/biosimilar-medicines-overview. Last accessed: January 2020
- 2. European Medical Agency. Biological Medicine. Available from: https://www.ema.europa.eu/en/glossary/biological-medicine. Last accessed: January 2020
- 3. The FDA. What are biologics? Available from: https://www.fda.gov/about-fda/center-biologics-evaluation-and-research-cber/what-are-biologics-questions-and-answers. Last accessed: January 2020
- 4. Cornes, P et al., 2019. Variability of Biologics and its Impact on Biosimilar Development. EMJ Reviews. Available from: https://www.emjreviews.com/hematology/symposium/variabili-ty-of-biologics-and-its-impact-on-biosimilar-development/. Last accessed: January 2020
- 5. Leung LK et al. What do oncologists need to know about biosimilar products? Chin J Cancer. 2016;35(1):91
-
6. European Medicines Agency. Biosimilars in the EU. Available from:
https://www.ema.europa.eu/en/documents/leaflet/biosimilars-eu-information-guide-healthcare-professionals_en.pdf.Last accessed: January 2020 - 7. GaBI. 2012. Small molecule versus biological drugs. Available from: http://www.gabionline.net/Biosimilars/Research/Small-molecule-versus-biological-drugs. Last accessed: January 2020
- 8. Tkaczuk, K.H.R. and Jacobs, I.A., 2014, April. Biosimilars in oncology: from development to clinical practice. In Seminars in oncology (Vol. 41, pp. S3-S12). WB Saunders.
- 9. Issacs J, Gonçalves J, Strohal R et al., The biosimilar approval process: how different is it? Considerations Med. 2017;1:3-6.
- 10. Vulto AG, Jaquez OA. The process defines the product: What really matters in biosimilar design and production? Rheumatology (Oxford). 2017;56(Suppl 4):iv14-29.
- 11. Samsung Bioepis. Data on File.
- 12. Samsung Bioepis. Data on File.
- 13. The FDA. Scientific Considerations in Demonstrating Biosimilarity to a Reference Product Guidance for Industry. Available from: https://www.fda.gov/media/82647/download. Last accessed: January 2020
- 14. The EMA. Guideline on similar biological medicinal products containing monoclonal antibodies – non-clinical and clinical issues. Available from: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-monoclonal-antibodies-non-clinical_en.pdf. Last accessed: January 2020




