SCIENCE & TECHNOLOGY
Our R&D Expertise
A fresh perspective on biosimilar development
Continuous innovation and advanced platform optimization has allowed us to increase the speed of medicine delivery and raise the bar for medicine quality and supply, while still implementing rigorous control over quality.
Process Modeling and Simulation for Manufacturing
Scale-up is an important part of biosimilar development where stringent quality control is required. Even a small change in temperature or other cell culture conditions can affect the quality of biosimilars during scale-up from lab-scale to commercial scale. At Samsung Bioepis, we have established a robust scale-up platform based on multiple computer simulations and extensive experiments. During the development stage, our internal review committee assess the quality of biosimilars at each stage of development. For commercial production, we closely monitor the biosimilar quality within tight standards to reduce batch-to-batch variations.
More Precise Analytical Method
When developing biosimilars, it is important that we understand all the key scientific aspects of the reference medicine so we analyze its characteristics from multiple angles, using up to 80 methods. We need to understand how the medicine reacts biologically and physicochemically when it enters a human body, and how the medicine functions when it identifies target cells. Since biologics are made from living cells, they naturally have minor variations from one batch to another. To fully understand the reference medicine which may have batch-to-batch variations, we source a large quantity of the reference medicine.
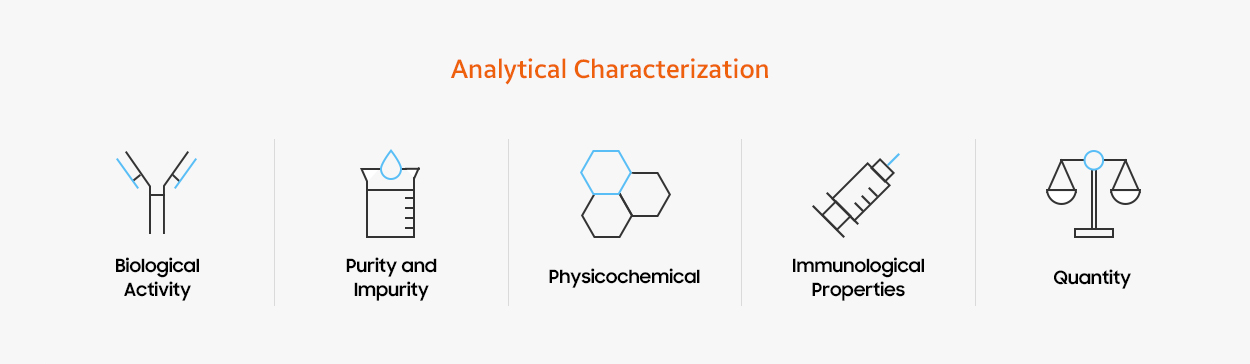
- Biological activity
- Purity and impurity
- Physicochemical properties
- immunological properties
- quantity
- Samsung bioepis - 1st
- Competitor A - 2nd
- Competitor B - 3nd
- Competitor C - 4nd
We use state of the art technologies to assess Critical Quality Attributes (CQAs).

Developing Medicines to Meet Unmet Needs
Patient safety and convenience is at the core of our biosimilar development. Based on our deep understanding of patients' needs, we endeavor to find innovative ways to improve patient convenience while ensuring safety. By establishing the conditions for protein stabilization, we have developed a formulation that shows superior stability compared to the reference medicine and we produce quality-assured products through aseptic process with excellent process control. In addition, considering patient convenience, we have developed a device that is easy to use, convenient and reliable for the delivery of medicines.
Quality by Design and Risk Management
One example of process innovation we use is Quality by Design (QbD). This is a systematic approach where the biosimilar development process is designed with pre-determined goals based on a risk assessment. In order to set the quality goals, we implement a rigorous analysis of the reference medicine so that Critical Quality Attributes (CQAs), or the characteristics that determine the quality of a biosimilar, can be determined. Failure Mode Effects Analysis (FMEA) is a risk management approach based on scenario planning and simulations. Based on knowledge gained from previous development projects, we built a robust mitigation strategy that allows us to plan for possible risks and minimize failures at each stage of the development process.
Tollgate System
Tollgate is a quality control system which allows us to assess whether quality goals are met at each inflection point. Only the highest-quality molecule that surpasses the quality standards can move up to the next stage of scale-up process.
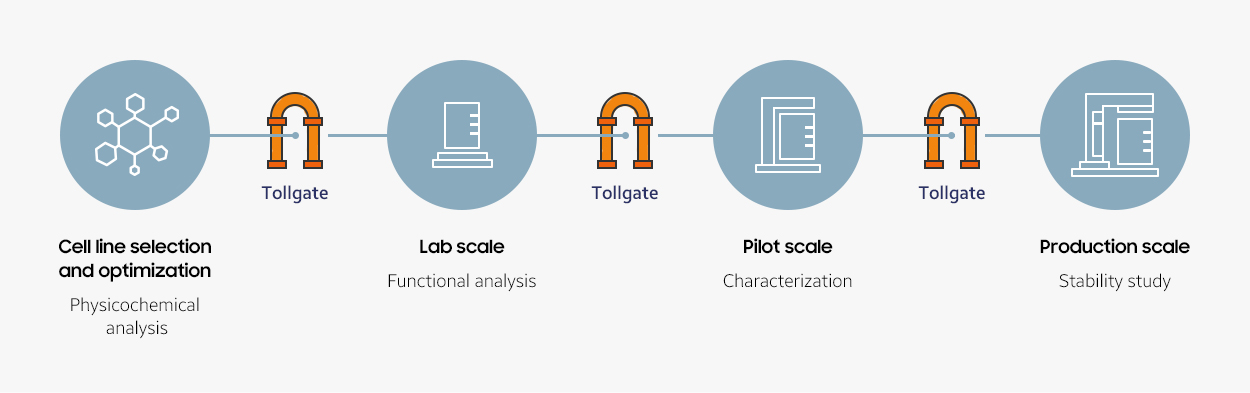
- 1. Cell line selection and optimization
- 2. Lab scale
- 3. Pilot scale
- 4. Production scale
Characterization + Stability study



