Our Stories
Our Stories

[Re:Access] The Future of Healthcare Needs Biosimilars
Welcome back to Re:Access, a newsletter series by Samsung Bioepis that Rethinks biosimilars as the answer to future healthcare Access.
In this issue, we explore how biosimilars sustain and strengthen healthcare systems through the “virtuous cycle.” But can it last?
The Virtuous Cycle
Healthcare systems benefit from biosimilars through the “virtuous cycle.”
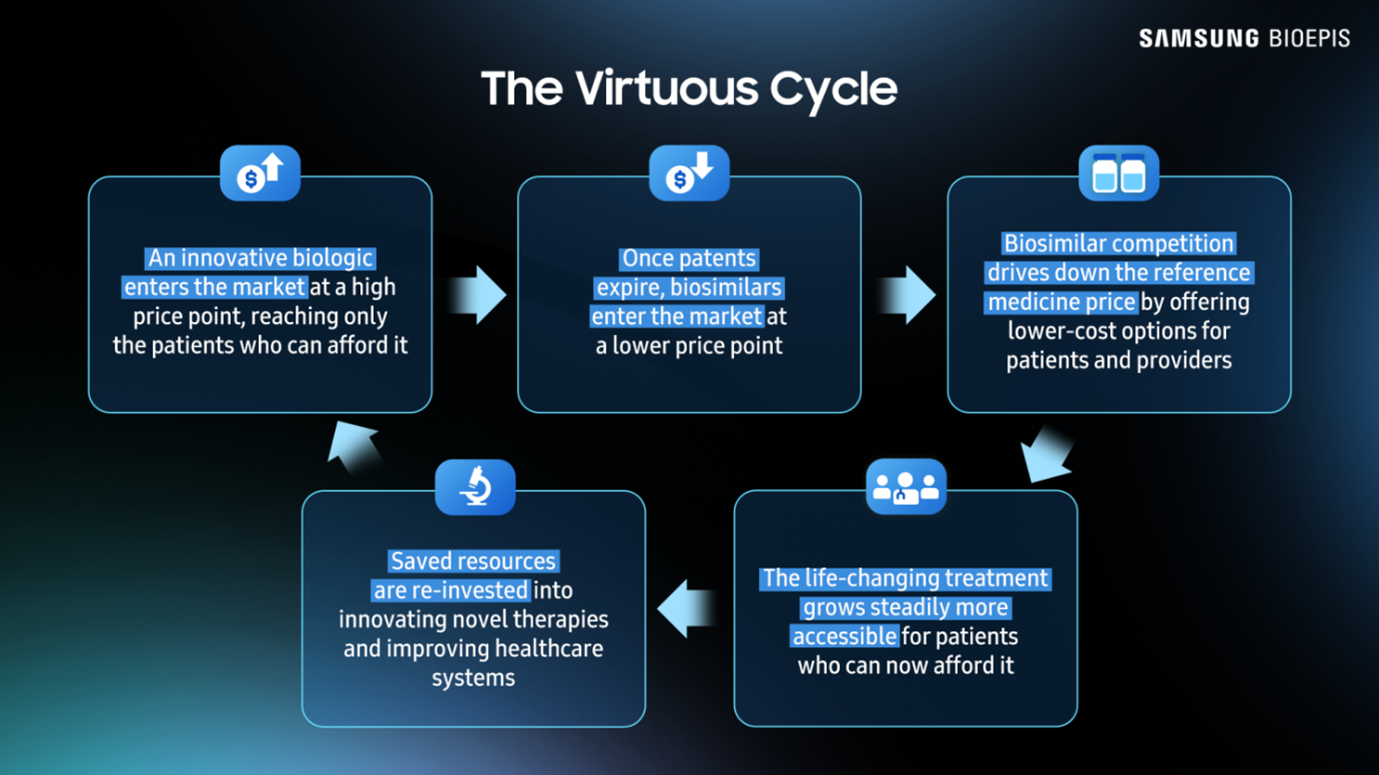
Biosimilars allow more patients to be treated with lower-cost alternatives to branded biologics:
● The US has seen $56 billion in cost-savings from using biosimilars since 2015. Biosimilars have been used in nearly 3.3 billion days of patient therapy, with biosimilar competition making over 460 million incremental days of patient therapy possible in the US.
● Across Europe, biosimilars have delivered €56 billion in cost-savings for healthcare systems and a total of 6.9 billion treatment days from 2013 to 2024.
The freed-up resources also create more headroom for innovation. For instance:
● Several regions in Italy reallocate 50% of biosimilar cost-savings to support 20% of the budget for breakthrough medicines.
● From 2019 to 2022, the National Health Service in England saved €474 million in savings from prescribing antirheumatic biosimilars, partially enabling €808 million in funding for access to cutting-edge treatments through the Innovative Medicines Fund.
These reinvestments clearly show the virtuous cycle in action: biosimilar uptake reduces costs, widens patient access, and funds innovation.
The problem? There may be no virtuous cycle in our future due to a significant gap of biosimilars in the pipeline.
Why is the Virtuous Cycle at Risk?
Due to steep price discounts, many biosimilar developers have failed to gain meaningful market share. As developing a biosimilar is costly and time-consuming, such market uncertainty has led to more manufacturers reevaluating whether they should continue investing in biosimilars.
In the UK, biosimilars have struggled to gain uptake as originator products are still preferred. In the US, originator products dominate formularies. As formularies are designed to favor more expensive products for most insurers and pharmacy benefit managers, providers do not have a financial incentive to prescribe lower-cost biosimilars. The situation in the US is unlikely to change unless payers and providers implement biosimilar-first strategies.
As Tom Newcomer, Head of US Commercial Operations at Samsung Bioepis, puts it: “Will biosimilar companies keep investing in biosimilars for these products if there’s no guarantee of uptake in the market?”
Inaction Risks the Sustainability of Healthcare Systems
So, what can be done to encourage biosimilar uptake?
Adam Levysohn, Head of Commercial Strategy Europe at Samsung Bioepis, recalls a conversation he once had with a leading rheumatologist in Germany. While the rheumatologist understood biosimilars were just as safe and efficacious as their originators, he refused to prescribe them as he was “not getting anything out of it.”
To counter that line of thinking, Adam supports offering physicians financial incentives for prescribing biosimilars. For example, gain-sharing has worked successfully in Southampton, UK, where biosimilar penetration rose when physicians and wards were given extra staff or new equipment to financially assist with the switch. The initial cost of planning these incentives can be offset by long-term savings.
“These physicians who were thinking that there was no benefit to prescribing biosimilars actually saw the benefit all of a sudden, because it had something in it for them as well,” Adam explains, highlighting the Southampton case as one that kickstarted the gainsharing system. “They could prescribe more freely, healthcare systems had more supply opportunities, and so on.”
When Biosimilars Thrive, So Do Healthcare Systems
Policy reforms that effectively encourage biosimilar uptake would ensure our healthcare systems reap the benefits of the virtuous cycle for years to come. Building regulatory and market frameworks for biosimilars with a forward-thinking view will help secure a resilient future for patients and healthcare systems.
As Adam states: “The more biosimilars are being used, the more they save. And the more biosimilars are used, the more it does good for us in the industry.”
Read more about our take on the current condition of the biosimilar market, and how it may affect the future of healthcare systems here.





