Our Stories
Our Stories

[Re:Access] The Future of Healthcare Needs Biosimilars
Rethinks biosimilars as the answer to healthcare Access.
Re:Access explores the role biosimilars play in expanding healthcare access around the world. We unpack the challenges surrounding biosimilar adoption through key insights, industry voices, and forward-thinking policy perspectives — and spotlight the urgent need for sustainable solutions.
Our first issue identifies a growing industry concern that this newsletter series will continue to shed light on: the biosimilar void.
What is the Biosimilar Void?
Thoughts of “The Void” keep Gillian Woollett, Head of Regulatory Strategy and Policy at Samsung Bioepis, up at night.
The concept ‘Biosimilar Void’ was introduced by IQVIA in a report a couple of years ago, referring to how there are no biosimilars in development for most biologics that are facing loss of exclusivity (LoE) in the future.
“This
is where we're seeing collectively a lot of companies are not persisting with
biosimilar development.,” Gillian says. “How can we help solve the pipeline problem
honestly in the next three to seven years if 90% of the biologics have no
biosimilars in development at all?”
But
what does a future without biosimilars look like? Why is this void so
concerning?
How
Biosimilars Benefit Healthcare Systems
A
future without biosimilars is concerning for several reasons. For one, such a
future lacks the benefits they bring.
Adam Levysohn, Head of Europe Commercial
Strategy at Samsung Bioepis, is confident that biosimilars have demonstrated
their worth. “From where we were in 2014-2015, to where we are today, there is
no question that biosimilars have proven their value proposition. They have
benefited all stakeholders at the same time.”
Biosimilars
bring transformative benefits to healthcare systems:
● Cost-savings: In the last decade,
biosimilars have delivered €56 billion in cumulative savings across Europe.
● Broader
patient access:
Through biosimilars, patients gain wider and earlier access to biologic
treatments.
● Supply
security: Diversifying
the supply of biologic medicines improves supply chain resilience and reduces
the risk of supply shortages.
However,
without investment and strategic policy shifts, the biosimilar void threatens
to erase these gains. In Europe alone, €15 billion in savings—25% of LoE
opportunities—could be lost by 2032 due to this gap.
Tackling the Biosimilar Void in Europe
So why
are manufacturers hesitant to enter the biosimilar market? For one, the process
from development to approval is time-consuming and costly. When returns on
investment are impeded by regulatory inefficiencies and external price
controls, the decision to enter the market can seem risky.
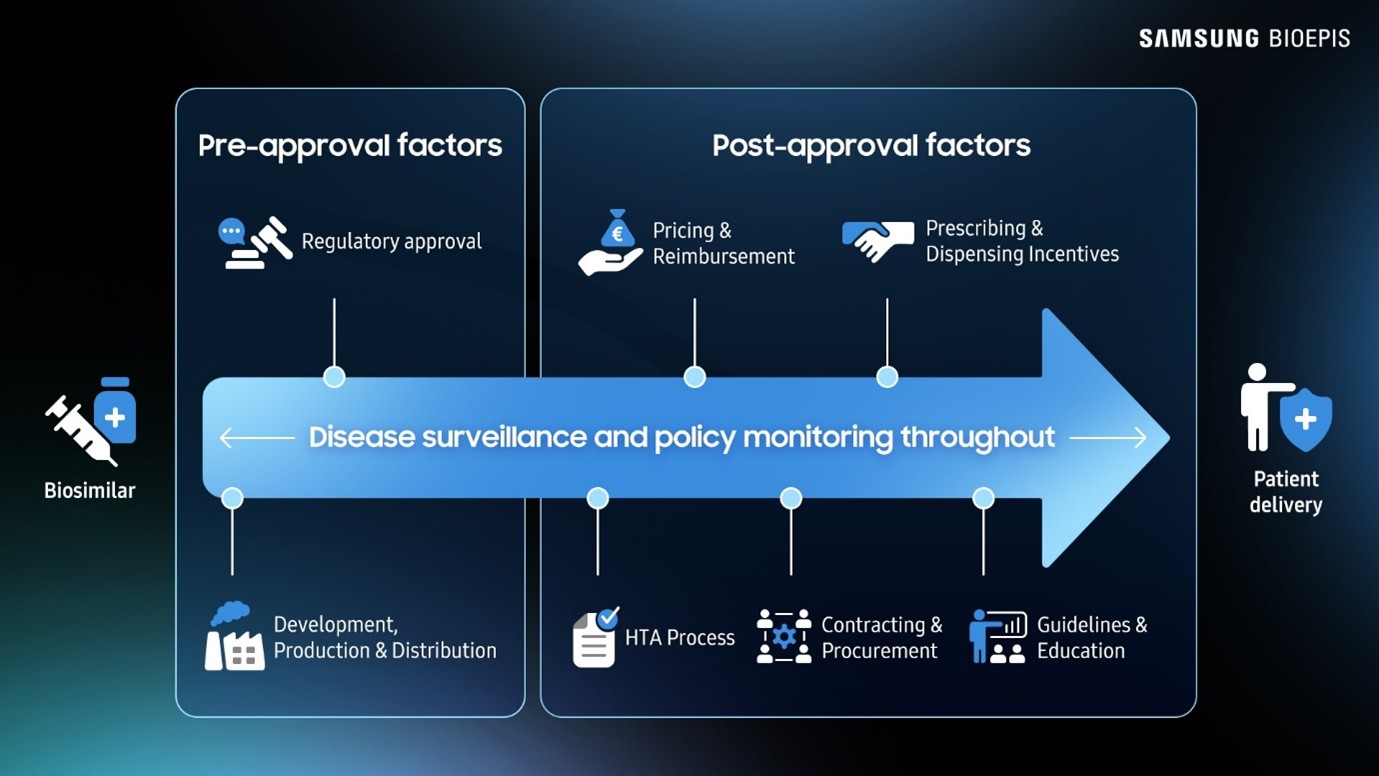
[The current post-launch policy
framework in Europe presents structural challenges that threaten market
sustainability.]
Though biosimilars do provide more affordable alternatives to reference products, discounts that cut too deep into profitability make the business unviable – especially when development costs are so steep.
“Prices
are dropping faster than I had anticipated,” Adam notes. “You see some
governments starting to implement policies that are not necessarily helpful. I
wonder whether the sustainability of the market is at risk here.”
To
sustain the influx of saving opportunities and improved patient access to
treatments, thoughtful policy reform is required to facilitate collaboration
among stakeholders.
Our
proposed policy changes that will be key to driving progress include:
1.Simplifying
and tailoring health technology assessment (HTA) processes to support timely
and value-based decisions.
2. Implementing
fair biosimilar pricing rules and controls at launch.
3. Providing
exemptions for biosimilars from wider cost-containment policies.
4. Updating
tendering processes to adequately consider biosimilars and support their
competition.
5. Encouraging
prescription of high-quality biosimilars while preserving stakeholder autonomy.
6. Increasing
education for key stakeholders to support understanding of biosimilar value.
Unlocking
the Full Potential of Biosimilars for Future Healthcare Access
Unless
policies are adapted to integrate biosimilars more effectively into healthcare
systems, the biosimilar void will only grow bigger. It will take concerted
efforts from different stakeholders and jurisdictions to streamline processes
and incentivize the continued development of biosimilars.
“These are marvelous medicines that are changing people’s lives,” said Gillian. But under current conditions, biosimilars cost too much to develop, take too long to be approved, and inevitably enter a reimbursement system built to keep prices high. “After all, biosimilars are themselves innovative and a crucial part of the virtuous cycle that gives headroom for the next generation of innovator medicines.” The virtuous cycle is critical to new medicines, and for it to prosper biosimilars are vital, she concludes.
Read more about our take on how to solve the biosimilar void in Europe here.
Follow us for updates on how pricing, policies, and uptake can shape the biosimilar market’s sustainability.





